|
DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) play fundamental roles in carrying genetic information in all life on Earth. DNA is a macromolecule that has the structure of a double helix that looks like a twisted ladder in which the rungs of the ladder consist of matching pairs of nucleobases (Figure 1). RNA is similar to DNA, but has only one strand of the helix and uses a slightly different set of nucleobases.
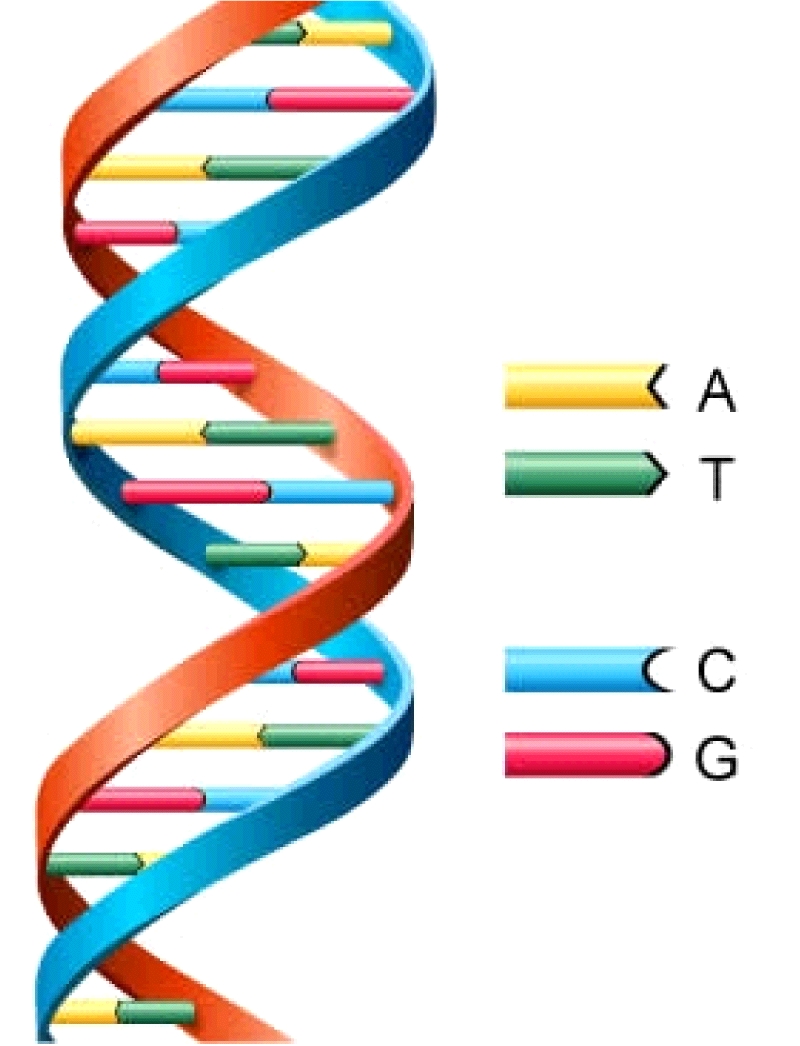
Figure 1: Schematic showing the molecular structure of DNA, a double helix linked
by pairing nucleobases: adenine (A) with thymine (T), and cytosine (C) with guanine (G).
There are a total of 5 nucleobases in DNA and RNA. These are cytosine, guanine, adenine (found in both DNA and RNA), thymine (found only in DNA), and uracil (found only in RNA). In DNA, adenine pairs with thymine, while cytosine pairs with guanine. In RNA, the thymine is replaced with uracil.
Uracil, cytosine, and thymine share a similar structural backbone, the molecule pyrimidine, a six-membered aromatic ring with two nitrogen atoms in the ring (Figure 2). The nucleobases uracil, cytosine, and thymine are essentially pyrimidines to which various combinations of chemical side groups (=O, –CH3, and –NH2) have been added.
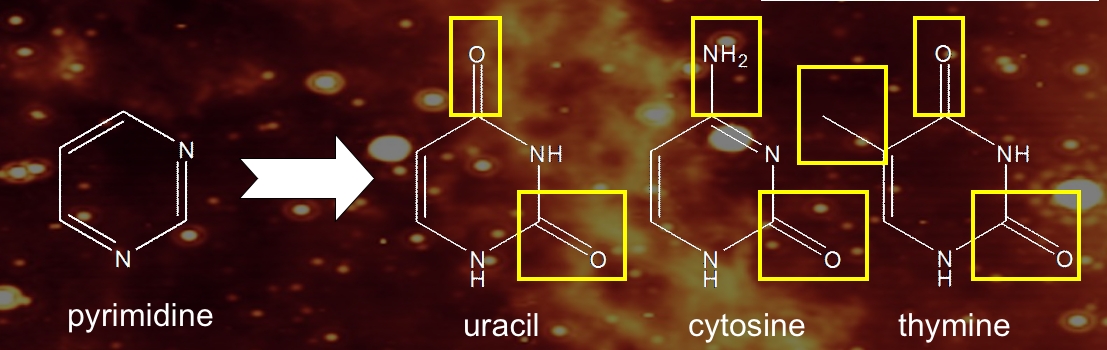
Figure 2: Chemical structures of pyrimidine and the three pyrimidic nucleobases uracil, cytosine, and thymine.
The other two nucleobases, adenine and guanine, share the structural backbone of the molecule purine, a six-membered aromatic ring fused to a five-membered aromatic ring with two nitrogen atoms in each ring (Figure 3). The nucleobases adenine and guanine are essentially purines to which various combinations of chemical side groups (=O and –NH2) have been added.
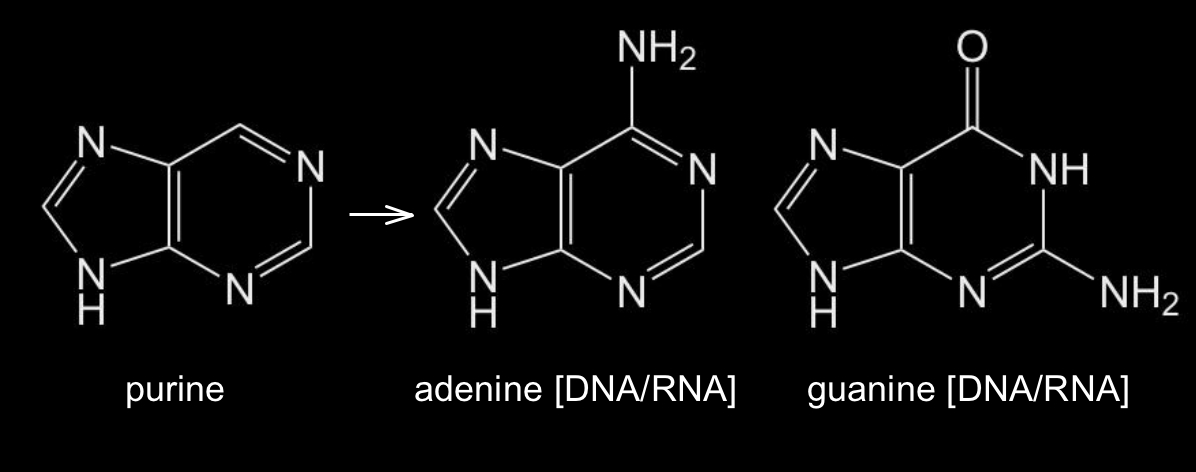
Figure 3: Chemical structures of purine and the two puric nucleobases adenine and guanine.
The Presence of Pyrimidine and Purine in Space
Pyrimidine and purine have yet to be detected in interstellar space, but they are seen in meteorites. These molecules are observed to be enriched in deuterium, proving they have an extraterrestrial origin. The origin of these molecules is not currently understood, although N-bearing aromatic species are expected to form in the stellar outflows of carbon stars. Thus, these aromatic species may be available to participate in the same sort of chemistry that can modify other aromatic species like polycyclic aromatic hydrocarbons (PAHs).
The Chemistry of Pyrimidine in Space
Pyrimidine, like PAHs, is a relatively non-volatile compound, so that in space it is expected to freeze out into the ices that coat cold dust grains in dense interstellar clouds. The ices in dense interstellar clouds are dominated by the molecule H2O, but also contain many other species like methanol (CH3OH), carbon monoxide (CO), carbon dioxide (CO2), ammonia (NH3), methane (CH4), formaldehyde (H2CO), as well as other simple species.
Our laboratory work has shown that when PAHs are exposed to high-energy radiation in these types of ices, a host of new molecular compounds consisting of PAHs with their edges modified by the addition of various chemical side groups like =O, –OH, –NH2, –OCH3, –COOH, –CH3, and –CN are produced (see PAH-related compounds).
Interstellar ices are generally dominated by the molecule H2O. When pyrimidine is photolyzed in H2O ices, the dominant reactions that we observe are O-atom addition reactions, just as is the case when normal PAHs are irradiated in H2O-rich ices. One of the primary products that is produced when one O atom is added is 4(3H)-pyrimidone, and the most favorable product that is produced when two O atoms are added is the nucleobase uracil (see Figure 4).
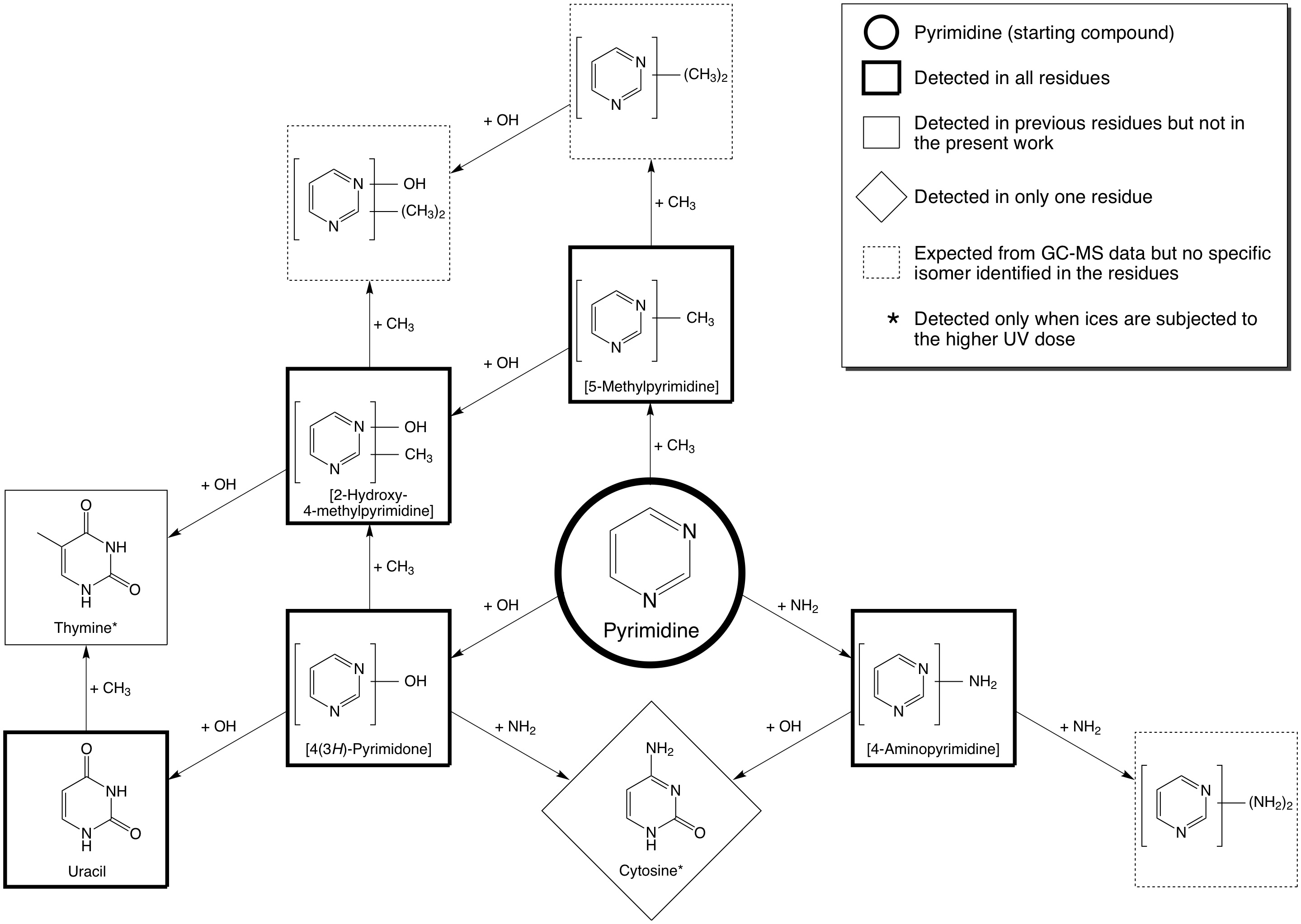
Figure 4: The addition of –OH, –NH2 , and –CH3 groups
from H2O, NH3, and CH4 ices, respectively, to pyrimidine results in the formation of a
wide variety of products which include the nucleobases uracil, cytosine, thymine.
The production yield of thymine is however lower than that of uracil and cytosine.
Similar experiments in which simple N-bearing molecules like NH3 (ammonia) are present show that –NH2 groups can be added to pyrimidine, and that one of the products is the nucleobase cytosine (see Figure 4). The addition to a methyl (–CH3) group, together with the addition of two O atoms, to form thymine (the third pyrimidic nucleobase) also occurs (see Figure 4), but is far less efficient. The first reason for this is because the formation of thymine requires the addition of three groups to a molecule of pyrimidine, meaning that more steps are necessary for its formation, and therefore its yield is expected to be lower than for uracil and cytosine, which only require the addition of two groups. The second reason is because the addition of a –CH3 group is less efficient than the addition of an –OH or –NH3 group, and because H2O is the most abundant ice in the matrix and –CH3 group addition is outcompeted by the addition of –OH groups.
This result may have had important implication in the origins of genetic material on the primitive Earth, as thymine is only used in DNA, while uracil is used in its place in RNA. Indeed, the low formation yield of thymine compared with uracil may have favored the use of uracil and thus RNA as the first material to store genetic information. This scenario is consistent with the ‘RNA World’ hypothesis, in which RNA is believed to have been used first as genetic material and as enzymes when life emerged, while DNA was a later addition to the biomolecular inventory.
The Chemistry of Purine in Space
Similarly to pyrimidine, the UV irradiation of purine in ices that contain H2O and NH3 lead to the addition of chemical groups to the peripheral carbon atoms of purine. This leads to the formation of the two puric nucleobases adenine and guanine (see Figure 3), as well as several other isomers and related species, such as hypoxanthine and isoguanine, most of which have been detected in primitive meteorites.
In these experiments, it was demonstrated that the formation yield of guanine is significantly lower than that of adenine and hypoxanthine. Interestingly, hypoxanthine has been proposed as a substitute to guanine to pair with cytosine in primitive versions of DNA, which indicates that hypoxanthine may have played an important role at the origin of the genetic code.
What is the Astrobiological Significance of the Formation of Nucleobases in Space?
The production of nucleobases by the irradiation of ices with small amounts of pyrimidine and purine under astrophysically relevant conditions supports another family of molecules of astrobiological significance that may form in space and be delivered to the surfaces of newly formed planets. Like amino acids, sugar derivatives, amphiphiles, and quinones and other PAH-related compounds, nucleobases produced in space may therefore have played a role in the formation, and subsequent evolution, of life on Earth and elsewhere in the universe.
For more detailed information and reviews on our laboratory work on nucleobases and related products made during the photoprocessing of aromatic molecules in ices, see:
Materese, C. K., Nuevo, M., McDowell, B. L., Buffo, C. E., & Sandford, S. A. (2018). The Photochemistry of Purine in Ice Analogs Relevant to Dense Interstellar Clouds. Astrophys. J. 864, 44 (6 pp).
Materese, C. K., Nuevo, M., & Sandford, S. A. (2017). The Formation of Nucleobases from the Ultraviolet Photo-Irradiation of Purine in Simple Astrophysical Ice Analogs. Astrobiology 17, 761–770.
Nuevo, M., Materese, C. K., & Sandford, S. A. (2014). The Photochemistry of Pyrimidine in Realistic Astrophysical Ices and the Production of Nucleobases. Astrophys. J. 793, 125 (7 pp).
Materese, C. K., Nuevo, M., Bera, P. P., Lee, T. J., & Sandford, S. A. (2013). Thymine and Other Prebiotic Molecules Produced from the Ultraviolet Photo-Irradiation of Pyrimidine in Simple Astrophysical Ice Analogs. Astrobiology 13, 948–962.
Nuevo, M., Milam, S. N., & Sandford, S. A. (2012). Nucleobases and Prebiotic Molecules in Organic Residues Produced from the Ultraviolet Photo-Irradiation of Pyrimidine in NH3 and H2O+NH3 Ices. Astrobiology 12, 295–314.
Nuevo, M., Milam, S. N., Sandford, S. A., Elsila, J. E., & Dworkin, J. P. (2009). Formation of Uracil from the UV Photo-Irradiation of Pyrimidine in Pure H2O Ices. Astrobiology 9, 683–695.
Elsila, J. E., Hammond, M. R., Bernstein, M. P., Sandford, S. A., & Zare, R. N. (2006). UV Photolysis of Quinoline in Interstellar Ice Analogs. Meteorit. Planet. Sci. 41?, 785–796.
Bernstein, M. P., Mattioda, A. L., Sandford, S. A., & Hudgins, D. M. (2005). Laboratory Infrared Spectra of Polycyclic Aromatic Nitrogen Heterocycles: Quinoline, and Phenanthridine in Solid Argon and H2O. Astrophys. J. 626, 909–918.
Bernstein, M. P., Moore, M. H., Elsila, J. E., Sandford, S. A., Allamandola, L. J., & Zare, R. N. (2003). Side group Addition to the PAH Coronene by Proton Irradiation in Cosmic Ice Analogs. Astrophys. J. 582, L25–L29.
Bernstein, M. P., Elsila, J. E., Dworkin, J. P., Sandford, S. A., Allamandola, L. J., & Zare, R. N. (2002). Side group Addition to the PAH Coronene by UV Photolysis in Cosmic Ice analogs. Astrophys. J. 576, 1115–1120.
Bernstein, M. P., Sandford, S. A., Allamandola, L. J., Gillette, J. S., Clemett, S. J., & Zare, R. N. (1999). UV Irradiation of Polycyclic Aromatic Hydrocarbons in Ices: Production of Alcohols, Quinones, and Ethers. Science 283, 1135–1138.
Many of these can be downloaded from our Publications page.
|